In recent years, real-world data (RWD) and real-world evidence (RWE) are experiencing a renaissance in cancer treatment. However, real-world evidence comes with its own pros and cons, raising important questions. Why is RWE becoming more widely used, and what should you consider when using it in your oncology practice?
Clinical trial data is a pivotal aspect of cancer treatment. Clinical trials have long been a central guidepost for the approval and use of oncology therapies1, whether honing in on drug dosages, evaluating cancer treatments for safety, or determining effectiveness. However, such clinical trials are not without limitations.
RWD and RWE are advantageous in the oncology field, often filling the gaps left by the inherent faults of clinical trial data. Recent legislation2, such as the 21st Century Cures Act, aims to improve data sharing and use. This could translate to greater use of real-world evidence in the drug development cycle. With how rapidly healthcare advances today, real world evidence and real world data also help fill in gaps that clinical research has not yet answered with time intensive randomized controlled trials.

The Federal Drug Administration (FDA) defines real-world evidence3 as clinical evidence that provides information about a medical product’s benefit or risk according to real-world data. RWD4may be any health information gathered during clinical care, observational data, patient billing, and other electronic health records (EHR).
The use of RWE can support cancer treatment effectiveness and improved patient health status. Since RWE is gathered differently than randomized controlled trials, it holds certain advantages over the constraints inherent in clinical trials. RWE developed from real world oncology data provides a broader spectrum of information.
The problem with clinical trials
Clinical trials are still considered the most definitive way to test whether a new cancer treatment or medical product works. Yet, unfortunately, they present some limitations. For one, clinical trials are generally costly and time-consuming.
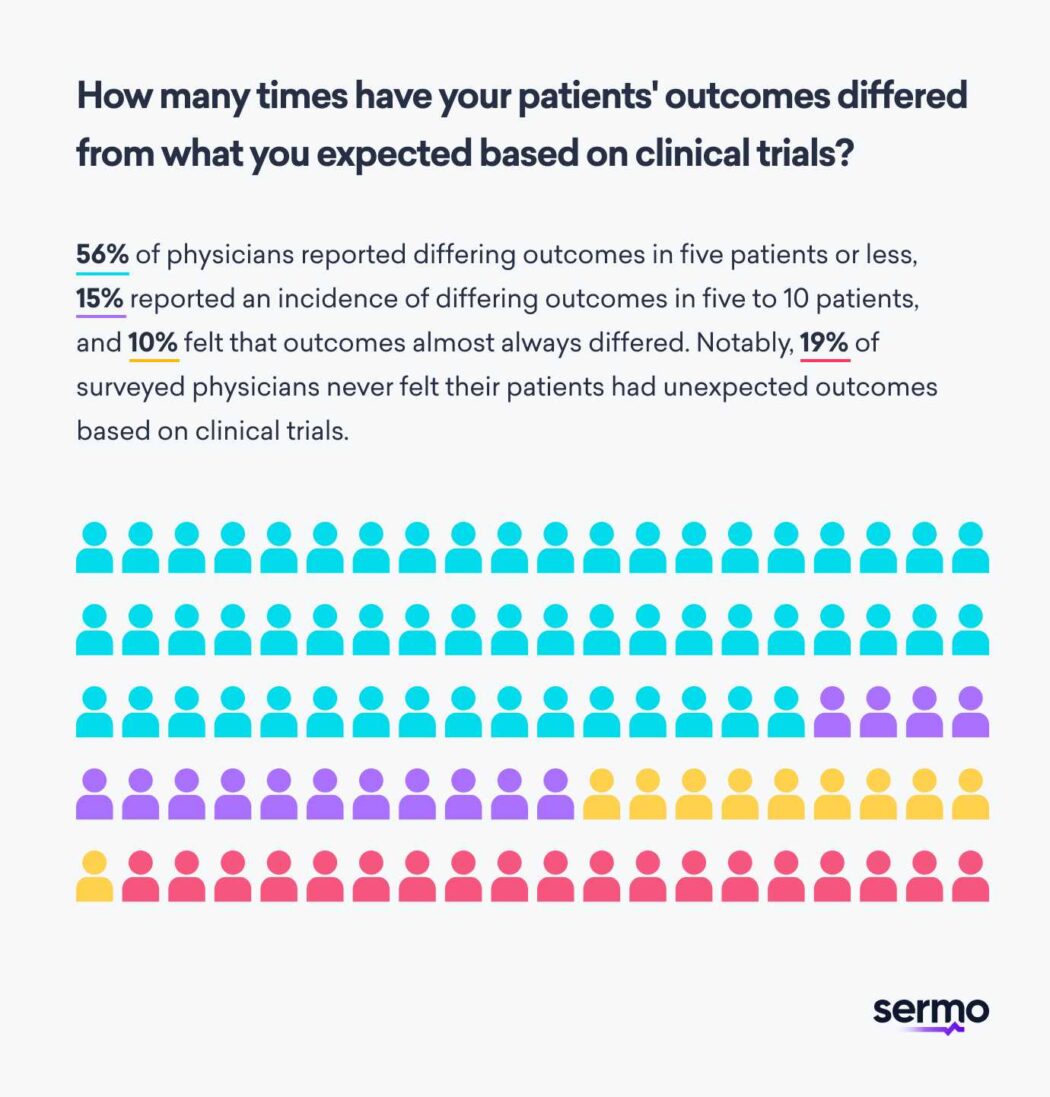
Additionally, clinical trials may not always accurately reflect the diverse patient population you see in the real world as an oncologist. In fact, many Sermo physicians surveyed reported that patient outcomes differed from what they expected based on clinical trial data5. 56% of physicians reported differing real world outcomes in five patients or less, 15% reported an incidence of differing outcomes in five to 10 patients, and 10% felt that outcomes almost always differed. Notably, 19% of surveyed physicians never felt their patients had unexpected real world outcomes based on clinical trials.
To compound the issues surrounding clinical trial data collected, your patients often come from all walks of life and varying socioeconomic statuses. Unfortunately, not every patient has the same access to healthcare. 40% of Sermo physicians surveyed reported that 10-25% of their patients have experienced worse cancer outcomes related to poor healthcare access. 10% felt that number was closer to 25-50% of their patients, 17% felt it was up to 75%, and 4% reported noting worse outcomes in more than 75% of their patients.
In addition, clinical trials6 are frequently performed at academic medical centers or other research-focused establishments. This does not match the real world medical settings of most patients. In a Sermo physician survey, only 13% of oncologists said that they provide care at academic research centers. The majority of surveyed physicians — 40% — provide care in an inpatient hospital setting. The second most common place was in outpatient clinic settings, with 26% of physicians caring for patients there. 13% cared for oncology patients in an NCI-designated cancer center, and 9% of physicians provide care in another setting not listed in the survey.
Barriers to entering clinical trials present a significant obstacle for many patients. Estimates reveal that only 2–4% of adult cancer patients participate in clinical trials. Many randomized controlled clinical trials (RCTs) are highly selective about who they accept. Data gathered in randomized clinical trials may not represent the range of patients seen in real-world clinical practice, where patients may exhibit lower tolerance, adherence, or worsening conditions. In a survey of Sermo physicians, 77% reported that their patients have had difficulty getting accepted to clinical trials based on factors such as comorbidities. Considering that such barriers exist, how can you be confident the study results apply to all of your patients?
In some instances, patients in clinical practice have fared worse than those that took part in an RCT. For instance, one analysis of patients7 with castration-resistant prostate cancer revealed that a regimen of docetaxel and prednisone yielded higher toxicity levels and worse survival rates in practice than within the clinical trial. Such results may be due to the patients chosen for RCTs lacking the heterogeneity of patients in clinical practice. This is where real-world evidence comes into play.

Real world evidence and real world data health data regulatory approval
The FDA and other government medical associations such as the European Medicines Agency are actively creating a real world evidence program to ensure data quality for routine clinical practice use. The FDA defines real world data (RWD) as “data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources and real world evidence (RWE) as the clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of RWD.
In alignment with the 21st Century Cures Act, the goal of the Oncology Center of Excellence Real World Evidence Program is to engage in evidence development modernization through scientific collaboration and policy development to advance the appropriate fit-for-purpose application of RWD to generate RWE for regulatory purposes.”
The value of real-world evidence in oncology health systems and patient care
RWE is essential because it’s pulled from real-world data. As RWD is not limited to certain populations, researchers can analyze cancer data from a variety of demographics, and not just clinical trials. In some cases, RWE backs up the efficacy8 of cancer treatments. And in other cases, RWE improves patient care and helps streamline care delivery.
For example, real-world data recently led to FDA approval of a biweekly dosing regimen9 of cetuximab for patients with metastatic colorectal cancer. Previously, patients needed to come in weekly for the drug. But RWE showed the efficacy of biweekly dosing, saving patients time and allowing them to more easily coordinate their appointments more easily.
There are also many instances of RWE reinforcing the results of clinical trials. When used appropriately, real-world evidence in oncology is a boon to cancer care. RWE can also shed light on several significant issues, like how treatment complexity, cost, and patient demographics affect patients’ compliance with their treatment regimens. The Oncology Center of Excellence Real World Evidence Program10 now seeks to advance the use of RWE in developing cancer treatments within a regulatory decision making context.
When data is collected from real-world sources, researchers can obtain a more comprehensive picture of treatment outcomes in clinical practice. Yet, despite its great value, RWE also has its negative side. Clinicians need to approach using real world evidence and real world data with some critical considerations.

Drawbacks of real-world evidence in cancer treatment
Just like any methodology, real-world evidence has its limitations. For instance, researchers face lower quality data collection and sources than those of clinical trials. On top of that, real world data lacks control groups, meaning it’s harder to compare treatment efficacy to any standard or other treatments.
Another limitation of real-world data is that oncologists sometimes under-report11 subjective patient symptoms in clinical practice. Therefore, information pulled from electronic health records pertaining to adverse events may be less accurate. On the other hand, participants in a clinical trial are carefully monitored for any subjective or objective adverse symptoms.
Data collected to generate real world evidence may be less credible than in a clinical trial as well. Outcomes research and data quality are less scrutinized by peers in real world evidence studies compared to clinical research. It’s also harder to establish cause and effect between a drug and an adverse event just from analyzing real world data.
How is real world evidence and real world data used in the cancer drug development process?
Healthcare systems, medical device companies, drug safety, and pharmaceutical companies are already using real world data and real world evidence in the drug development process.
Real-world evidence holds immense potential throughout the drug development life cycle. Real world evidences provide biopharmaceutical companies diverse opportunities to accelerate development, decrease clinical trial costs, and enhance the likelihood of technical and regulatory success.
- Early discovery. Oncology development is becoming more personalized and precise. It focuses on genomic alterations and signatures to identify biomarkers for therapeutic response and resistance. Real-world evidence using genomic sequencing and clinical data derisks early discovery, optimizing drug development. For example, a top ten pharmaceutical company recently utilized a clinico-genomic database with tumor sequencing information from over 2,000 patients with advanced nonsmall cell lung cancer to identify and characterize genomic profiles of patients with rapid progression or otherwise poor prognosis. These findings generate valuable insights to guide biomarker targets and support future targeted cancer drug development and drug safety.
- Trial design and feasibility. Targeted use of real world evidence (RWE) derived from electronic health records supports the design and optimization of clinical trials. RWE can help design a protocol that aligns with standard of care, assessing the impact of eligibility criteria on trial feasibility, and informing the selection of trial sites. A leading oncology drug developer, utilized real world evidence to design a dosing study protocol with strong external validity. They analyzed standard of care delivery in routine clinical practice for metastatic cancer patients, adjusting assessment frequency to align with real world patterns. The outcome? A protocol that is less burdensome for patients and investigators and reduces costs for the sponsor.
- Trial execution. In oncology, adopting external control arms can reduce trial size, duration, and cost. Randomizing patients for clinical trials to standard of care often delays recruitment, especially if early signals show significant benefit over standard treatment. The traditional control arm may be replaced by an “external” control derived from real world populations. This is particularly advantageous in rare malignancies where patient recruitment is challenging. External control arms can also be used as comparators for early-phase single-arm trials in oncology-related approvals, as observed in 11 of the last 12 approval processes through the FDA’s Breakthrough Therapy Designation Pathway. Pfizer recently validated such an approach for a subset of clinical trial patients with HER2-negative/hormone-receptor-positive metastatic breast cancer. The real world control arm replicated the clinical outcomes (progression-free survival and overall survival) observed in a subset of patients enrolled in a standard of care phase III control arm. Key questions on experimental design, external control arms, and regulatory approval processes are yet to be clarified.
See how companies are using real world data and real world evidence to boost physician engagement.

The future of RWE and oncology healthcare systems
Real world evidence that follows clinical trials is in-demand as it provides the best of both worlds — clinical trial data and evidence gathered in the field. RWE packs the most power when it’s backed by clinical trials, and vice versa. However, as an oncology practitioner, you may not always have access to pragmatic clinical trials that support medical claims data of real-world evidence. If you’re using RWE that’s not backed by clinical trials, it’s important to keep in mind the limitations of these datasets.
Joining a collaborative community like Sermo is an opportunity to tap into the invaluable experiences of likeminded colleagues. Through our interactive platform, doctors like you from all over the world can glean firsthand knowledge and share their wisdom with each other.
Sermo empowers you to make a difference by being an active participant in the conversation. Although clinical trials will remain the gold standard for assessing the efficacy of oncology care, there are many potential benefits from sharing patient reported outcomes, real-world observations, and evidence.
As the future grows nearer, RWE is becoming an integral part of evidence-based practice. We all want the best for our patients, and medical advances are happening at an unprecedented rate. In medicine, it will always be vital to look at things that did or didn’t work, both historically and more recently, to provide patients the most effective care possible. With Sermo, you can be part of a global community of physicians striving to do so every day.
Frequently Asked Questions on Real World Evidence and Real World data studies
What is real world data?
Real-world data (RWD) is defined by the FDA as “the data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources.”
Sources of real world clinical data include, but are not limited to: electronic health records and electronic medical records. Health care claims and billing activity. Medical product and disease registries. Data gathered from other sources such as health care mobile devices, wearables such a pedometers and smart watches.
What is real world evidence?
Real-world evidence (RWE) is the evidence derived from real-world data analysis. RWE refers to clinical evidence of the use, potential benefits, and risks of a medical treatment. Real world evidence is gathered by real world data analysis sourced from a variety of types of research. Real world outcomes research comes from observational studies (both prospective and/or retrospective), randomized controlled trials, large simple trials, routine clinical care, and pragmatic trials.
How does real world evidence and real world data relate to patient electronic health records and privacy?
Due to the vast amount of data collection and real world data mining of sensitive patient information, artificial intelligence has been leveraged to translate real world data into real world evidence. This creates additional challenges compared to traditional clinical trials.
RWE often contains sensitive patient information and electronic health record clinical data, such as their health situation, demographics, and behaviors. Protecting the privacy and confidentiality of patient data is crucial, especially when using AI algorithms that can identify individuals or infer sensitive information. Securing this health care data with an encrypted and anonymizer data extraction solution can help prevent privacy breaches.