Six million Americans are diagnosed with Alzheimer’s disease, but this number grows much higher when we account for the families and those close to patients that are also impacted. The good news: neurologists report on Sermo that cutting-edge therapies and treatments (like Leqembi) are showing results we can get excited about.
Lecanemab, sold under the brand name Leqembi, is a monoclonal antibody medication now approved for early Alzheimer’s disease. In response to this approval, Sermo ran a custom RealTime survey in January 2023 to US Neurologists to better understand the safety and potential of these drugs.
The goal was to gauge the industry’s opinions and awareness of these newly approved treatments. The survey included a total of 30 US Neurologists who personally treat patients with Alzheimer’s and are the primary physician treating 10+ patients per month. Here are the findings.
Leqembi perceptions
Many neurologists have been interested in Leqembi for some time and have already heard of its potential. In a separate Sermo study of 300 global physicians before Leqembi’s approval, 87% said they believe the new Alzheimer’s drug is promising and 84% believe this news should bring hope to their patients suffering from Alzheimer’s and their families.
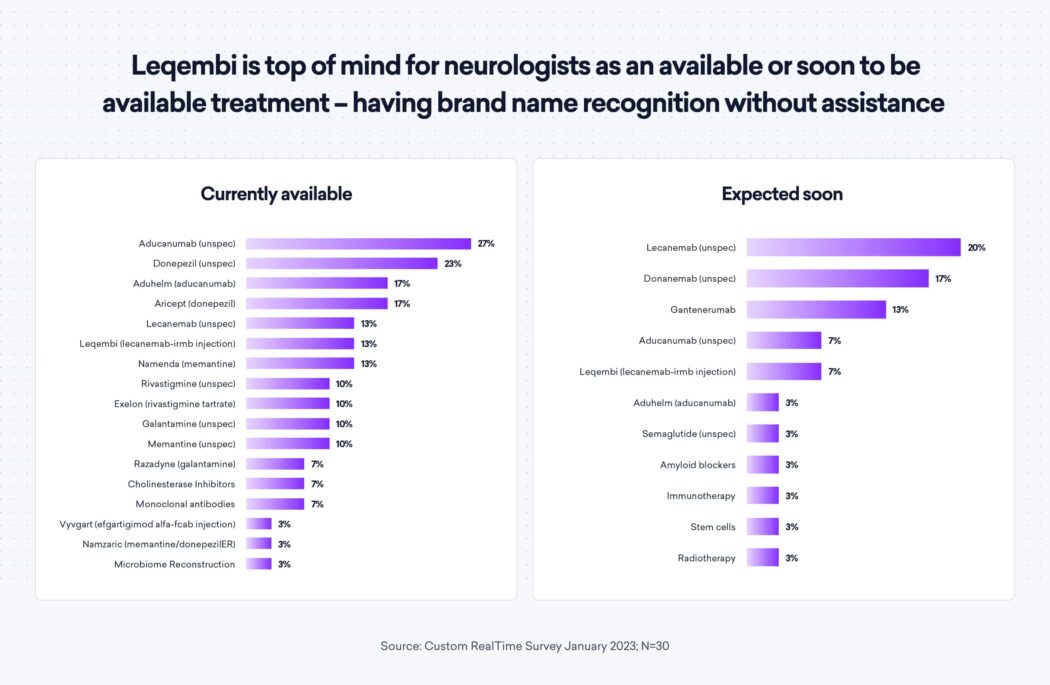
As a result of the recent approvals, Leqembi has received high brand recognition—in both unaided and aided awareness—as it is top of mind for neurologists as an available (or soon to be available) treatment. 60% of neurologists reported being fairly or well familiar with lecanemab as a treatment for early-stage Alzheimer’s in aided awareness.
Neurologist reservations
While physicians are impressed with the promise of lecanemab to slow the progression of early-stage Alzheimer’s disease, they have reservations about its safety. Additionally, there is some level of concern about patient compliance with the IV administration of lecanemab.
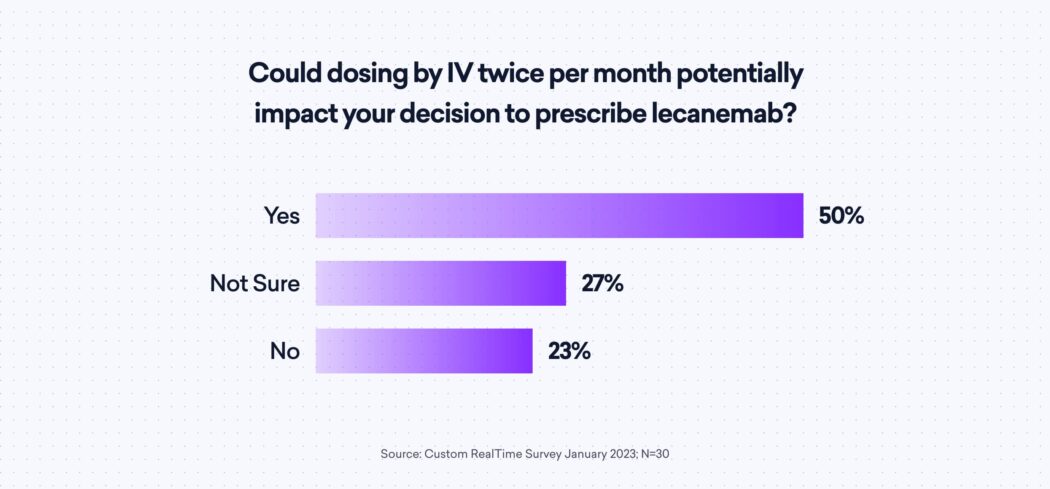
Some neurologists in the study see the formulary coverage being a critical component. They also shared mixed feelings about IV administration, even with 83% reporting having on-site IV administrative capabilities. The need for twice monthly IV dosings has 50% of surveyed neurologists agree that it would impact their decision; another 27% are unsure.
Finally, neurologists are concerned about patient access to this drug. Physicians surveyed are split 50/50 on if the drug is cost-prohibitive for prescribing to patients.
Most strongly agree, and expect, that more studies will follow to prove the drug’s benefits.
Meet the Sermo Alzheimer’s disease treater
Who are the Sermo Neurologists surveyed in this custom RealTime survey? Over half of Sermo physicians self-identify as “innovators” or “early adopters” when treating early-stage Alzheimer’s disease.
The Sermo Alzheimer’s Disease Treater strongly believes in early-stage management of Alzheimer’s disease and highly values treatments that prioritize the patient’s quality of life.
The point of view of the Sermo Alzheimer’s Disease Treater
- Strongly believe that early-stage management of Alzheimer’s disease is critical
- Highly prioritize treatments that offer the best quality of life for their patients
- Physicians are not too busy to keep up with new developments in Alzheimer’s disease
- Physicians value information about products in clinical development from medical journals, peers & conferences—online/virtual access is critical
—
On behalf of Sermo, thank you for checking out our blog post on Alzheimer’s disease. This is just one of the many examples of native engagement opportunities and insights available with RealTime studies on Sermo.
At Sermo, we turn physician experience, expertise, and observations into actionable insights for the global healthcare community. Engaging with more than 1 million HCPs across 150 countries, we provide physicians with a social platform that fosters impactful peer-to-peer collaboration & discussions about issues that are important to them and their patients. Sermo offers on-demand access to physicians via a suite of proprietary technology to provide business intelligence that benefits pharmaceutical, healthcare partners and the medical community at large.
Interested in learning more? Check back any time and follow us on Facebook, Twitter and LinkedIn for the latest and greatest in healthcare insights.
To explore our exclusive business solutions, please visit us at sermo.com/business or email us at business@sermo.com.