For those of us over age 55 years there is a lifetime risk of developing atrial fibrillation (AF) of 37%. And with AF comes a 5 fold risk of stroke which can be reduced by oral anticoagulant therapy.
Because up to 25% of AF is asymptomatic, investigators have recognized a need to screen for AF in high risk individuals with the goal of reducing stroke.
There is no consensus on 1) whether we should screen for AF, or 2) if we should, how should we do it, or 3) whether individual found by screening should be treated with oral anticoagulants just as we treat clinical AF patients.
Two studies presentations at the 2018 American College of Cardiology Meeting in Orlando earlier this month shed some light on the problem of subclinical AF.
The mHealth Screening To Prevent Strokes (mSTOPS) study took a novel approach to identifying and enrolling patients. Working with Aetna, invitations were sent by email or snail mail to over a 100,000 Aetna members who did not have a diagnosis of AF and who were over age 74 year or younger but with CHADS2 risk factors.
The members were directed to a website for information on the the trial and were guided through an enrollment process for informed consent. Half were randomized to early monitoring and half delayed monitoring at 4 months.
Immediate monitoring involved the patient being mailed a 14 day patch type monitor (Zio Patch, iRhythm Technologies Inc.) within 2 weeks of consenting to the trial. Patients in the delayed monitoring group received the patch 4 months after consent.
The primary endpoint was a new diagnosis of AF at 4 months, defined as greater than 30 seconds of AF by ECG or a new diagnosis listed on Aetna claims data.
Patients in the immediate or early monitoring group were 9 times more likely to have AF diagnosed than those in the delayed group (prior to them wearing the Zio).
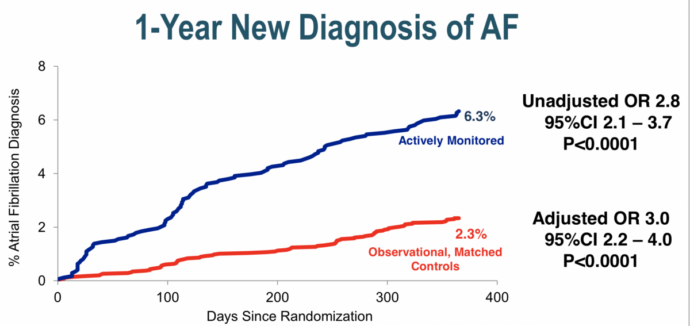
Diagnosis of AF in these participants were then compared to 5,310 observational controls matched for age, sex and CHADS-VASc score who did not undergo screening.
At the end of one year 6.3% of the monitored group had been diagnosed with AF versus 2.8% in the observational controls, a highly significant 3 fold increase in AF detection.
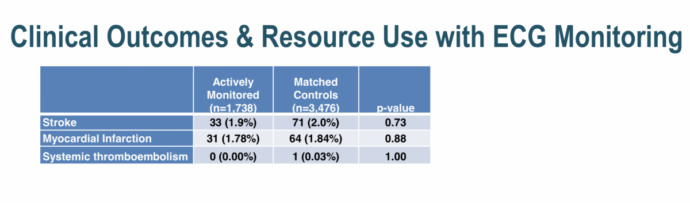
More patients in the monitoring group were started on therapy and had more testing done (at the discretion of their physicians who were informed of the results at the patient’s discretion).
There was no difference in stroke, MI or systemic thromboembolism at one year between the two groups but clearly this study was underpowered and nonrandomized so differences would have been unlikely and if present hard to interpret.
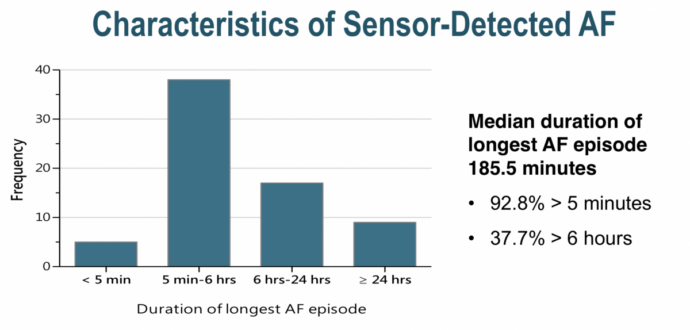
AF detected by screening and AF detected only by implanted devices share many similarities. For both, if we seek them we shall find them (one study showed in patients with no history of AF undergoing new pacemaker implantation during a mean follow-up period of 596 ± 344 days, 77 (29%) patients had at least one AF episode lasting ≥ 5 min)
At another session at the ACC18 two EP doctors debated whether a high CHADSVAS risk individual with 9 minutes of a atrial high rate episode (AHRE) of presumed AF detected by pacemaker should receive anticoagulation.
I ended up agreeing with the CON side of this debate for most patients because:
- device detected AF has a lower stroke risk (precisely how much unclear and related to CHADSVAS score but I’m going with 1/3) than clinically detected AF
- The ASSERT study only showed increased risk for >24 hours duration of device detected AF
- Unlike clinical AF we have no RCT data supporting treatment with OACS for DDAF
Ideally, patients like this with <24 hours of DDAF will be enrolled in one of two ongoing randomized trials which will give us the correct answer. Similarly, we desperately need randomized studies on the effects of screening for AF on long term outcomes.
Read Dr. Pearson’s previous post from this year’s ACC:
Login or join Sermo to contribute your opinions to this post.
Dr. Anthony Pearson is a clinical cardiologist and director of noninvasive cardiac imaging at St. Luke’s Hospital in St. Louis, Missouri. In his spare time he plays keyboards and guitar in the band, Dr. P and the Atherosclerotics. Blog: www.theskepticalcardiologist.com | Twitter: @skepcard