Over 47 million Americans have a “silent” build-up of amyloid plaques—high levels of amyloid plaques without any memory loss or cognitive impairments. Neurologists call this preclinical Alzheimer’s disease.
With new FDA-approved drugs like donanemab and lecanemab, Alzheimer’s treatments are expected to grow into a $30 billion industry in the next decade, potentially revolutionizing dementia care. To gain timely insights into Alzheimer’s disease, Sermo partnered with experts Dr. Murali Doraiswamy from Duke University School of Medicine and Dr. Lon Schneider from the University of Southern California to develop an in-depth survey.
Here’s what we found out:
Anti-amyloid therapies: a new standard of care
A significant majority of neurologists globally (72%) consider anti-amyloid therapies to be the standard of care for patients with mild cognitive impairment (MCI) or mild dementia due to Alzheimer’s disease. This marks a substantial shift towards these therapies. However this majority is much slimmer in the US where only 59% of neurologists considering them a standard of care versus 41% who do not – suggesting that patients would receive very different care depending on which neurologist they went to.

Diagnostic tools: the role of blood-based biomarkers
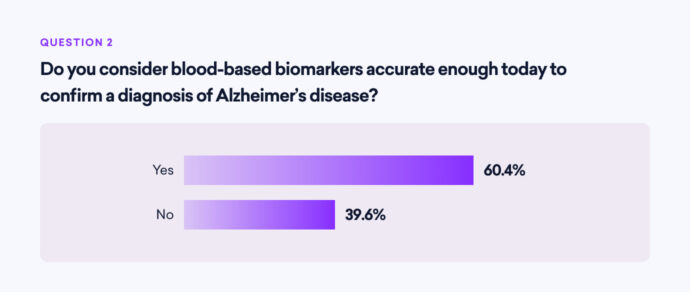
Blood-based biomarkers have gained traction as diagnostic tools for Alzheimer’s disease.
60% of the surveyed neurologists believe these biomarkers are accurate enough to confirm an Alzheimer’s disease diagnosis today. This is indicative of advancements in diagnostic technology, which may lead to earlier and more reliable detection of the disease. However, only 5% of neurologists viewed them as a marker of clinical benefit (more on this below).
Evaluating amyloid-targeting antibodies
When asked to evaluate the benefit-risk ratio of actively marketed amyloid-targeting antibodies, the responses were varied:
- 33% of neurologists favored Lecanemab
- 22% favored Donanemab
- 30% saw no difference between the two
- 15% were unsure
This diversity in opinion highlights the need for further comparative studies and real-world data to guide clinical decision-making.
Hippocampus volume and brain changes
Regarding Lecanemab and Donanemab’s ability to attenuate hippocampus volume loss compared to placebo, 73% of neurologists consider this a measure of clinical benefit.
However, there are concerns about these treatments leading to greater lateral ventricular volume enlargement and whole brain volume loss. While only 22% are seriously concerned about these side effects, 71% are mildly concerned, and 7% are not concerned at all.
Measuring clinical benefit
Determining the best measure of clinical benefit for treatments targeting MCI or mild AD dementia is crucial. More than 4 in 10 (44%) consider daily function (activities of daily living) the most important measure, followed by 34% cognitiom and 18% quality of life. Only 5% selected biomarker improvement, such as reduction of amyloid plaques on PET scans.
Safety concerns: ARIA risks
A significant concern among neurologists is the risk of Amyloid-Related Imaging Abnormalities (ARIA), which includes brain edema (ARIA-E), microhemorrhages, and superficial siderosis (ARIA-H). 71% view the risk for ARIA as a serious concern, specifically:
- 83% consider both ARIA-E and ARIA-H the most concerning
- 11% are most concerned about ARIA-H
- 6% are most concerned about ARIA-E

Use of anti-amyloid treatments in preclinical AD
The use of anti-amyloid treatments for cognitively unimpaired individuals with positive amyloid PET scans (preclinical AD) is a topic of debate:
- 53% of neurologists would use these treatments in such cases
- 47% would not
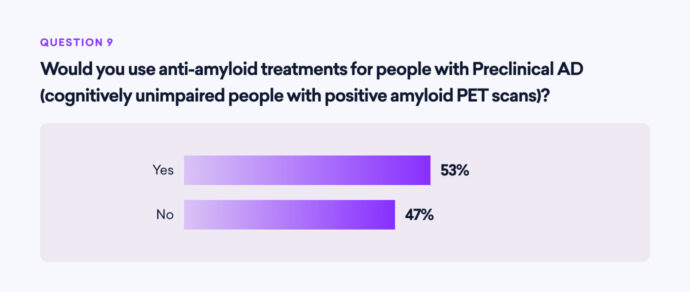
This split decision underscores the ongoing debate and the need for more evidence on the benefits and risks of early intervention.
Current treatment practices
Despite the advancements and approvals of anti-amyloid treatments, less than half (40%) of the surveyed neurologists have treated patients with Aducanumab, Lecanemab, or Donanemab. A majority (60%) have not yet incorporated these treatments into their practice, indicating either a cautious approach, skepticism, or limited access to these therapies.
Conclusion
Sermo’s survey of 268 neurologists provides a detailed snapshot of current perspectives on Alzheimer’s disease treatments. While there is a growing acceptance of anti-amyloid therapies as a standard of care, concerns about safety and varying opinions on clinical benefits remain. Continued research, real-world evidence, and comprehensive risk-benefit analyses are essential to guide the future of Alzheimer’s disease management.
Footnote
Murali Doraiswamy, MBBS, FRCP is professor of psychiatry and geriatrics at Duke University School of Medicine and a member of the Duke Institute for Brain Sciences, and serves as an advisor to Sermo.
Lon S. Schneider, MD, MS, is professor of psychiatry, neurology, and gerontology at the University of Southern California where he directs the USC California Alzheimer’s Disease Center
Methodology
Sermo conducted a 10-question survey among 268 global (limited to US and Europe) neurologist members of Sermo’s physician community on June 14, 2024. The survey was completed within 8 hours, focusing on neurologists’ opinions regarding Alzheimer’s disease trends, specifically the use of blood biomarkers and amyloid-targeting antibodies.
Participants were asked about their views on anti-amyloid therapies as a standard of care for mild cognitive impairment (MCI) or mild dementia due to Alzheimer’s disease, the accuracy of blood-based biomarkers for diagnosing Alzheimer’s, and the benefit-risk ratio of different amyloid-targeting antibodies. The survey also queried neurologists on their preferred measures of clinical benefit for treatments aimed at MCI or mild Alzheimer’s dementia, their concerns about ARIA (amyloid-related imaging abnormalities), and their views on hippocampus volume loss and brain volume changes as measures of clinical benefit. Key survey limitations include sampling bias, unmeasured confounding variables, and other factors.