In October of 2016 the Amplatzer PFO Occluder device (St. Jude Medical) was approved by the FDA for “percutaneous transcatheter closure of PFOs in patients, presumably between the ages of 18 and 60 who have had a cryptogenic stroke due to a presumed paradoxic embolism as determined by a neurologist and a cardiologist.”
At the 2018 ACC Meeting in Orlando last month I attended a session entitled PFO closure :state of the data to understand better which of my stroke patients would benefit from PFO closure (PC.)
Sanjiv Kaul reviewed the five RCTs the FDA considered in their decision.
The two most recent trials REDUCE and CLOSE both met their primary endpoints, a feat that was not achieved in three prior trials of PFO closure — CLOSURE I, PC, and RESPECT. Subsequent meta-analyses and longer-term data, however, have shown benefits for PFO closure, with a significant reduction of ischemic stroke.
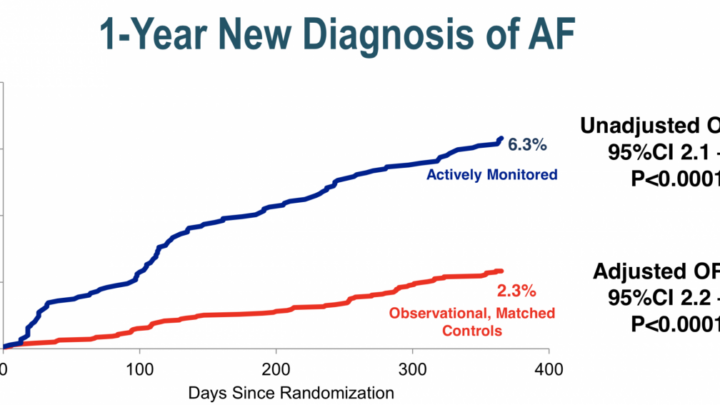
The most recent meta-analysis (DeRosa 2018) shows PC treatment superior to medical treatment with a relative risk of 0.42 for stroke but at the cost of a 4.69 fold increase risk of atrial fibrillation.
Kaul pointed out that current guidelines from the AHA/ASA, and Canadian and American Neurology Associations recommend against PFO closure in cryptogenic stroke patients without DVT.
The De Rosa meta-analysis concluded that 1000 patients treated for 2.7 years would result in the prevention of 31 strokes but with 33 excess cases of atrial fibrillation.
Post hoc analysis of the RESPECT extended follow up data (Saver JL et al, NEJM 2017;377:1022-32) that patients with larger shunt size, atrial septal aneurysm and “planned antiplatelet therapy” (as opposed to anticoagulant therapy) benefited most from PFO closure compared to medical therapy.
This afternoon results of the DEFENSE-PFO trial, were presented which sought to evaluate the benefit of PFO closure with the Amplatzter device in Korean cryptogenic stroke patients who had these putative morphologic markers for high risk PFO.
They enrolled patients who had PFO size by TEE >/=2mm, an atrial septal aneurysm, or hypermobility (>10mm septal excursion
I did not hear them define their criteria for atrial septal aneurysm.
At 2.8 years follow up there were no strokes in the 60 patients who had PFO closure versus 6 in the group on medical therapy.
We await the most current iteration of neurologic and cardiology guidelines in this area but for now consider PFO closure for youngish cryptogenic stroke patients with PFOs >/=2 mm, aneurysmal or hyper mobile atrial aneurysm after an exhaustive search which excludes other causes.
Read Dr. Pearson’s previous posts from this year’s ACC:
- ACC18: Pricey Praluent Proves Beneficial (sort of) Post ACS
- ACC.18: Subclinical Afib: Seek (with Zio) And Ye Shall Find
- ACC2018: HF with Normal EF: Another Drug Bites The Dust
Login or join Sermo to contribute your opinions to this post.
Dr. Anthony Pearson is a clinical cardiologist and director of noninvasive cardiac imaging at St. Luke’s Hospital in St. Louis, Missouri. In his spare time he plays keyboards and guitar in the band, Dr. P and the Atherosclerotics. Blog: www.theskepticalcardiologist.com | Twitter: @skepcard